Ford Parts Wiki | GM Parts Wiki
Home | Search | Browse
|
Technical Service Manual January 1975 |
|
Prev
 Next
Next
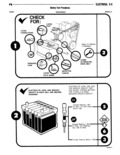
3 4 ELECTRICAL VI Frozen Eleclrolyte BATTERY MAINTENANCE A 3 4 charged automotive battery is in no danger of damage from freezing Therefore keep the batteries CAUTION Aiwa S 0b the t Poianti at a 4 charge or mm especially during winter Reveeed bmw cvmmtmsmay iam 0 M eww weather tor dwdes A battery in which the electrolyte is either slushy The NEGATIVE hnttery terrninni ie eenneeted te or frozen should be replaced Batteries with this condi the engine and te the fender inner nnneh tion depending on the severity of the freeze may it is very imnertnnt thnt the hettery he in n fully accept and retain a charge and even perform satisfac charged ennditien when e new enr ie deiivet ed The llY undelf a load WSP H V r fm 120 FO 50 continual operation of a partially charged battery days in service a reductron in capaeity and service life eenid shorten ite hre and resuitin renineement will become appamm as me lndmdual Plates 1 Fluid level in the battery should be checked periodi their actiw mat l l cally and replenished with distilled water if possible F i T cn ri However drinking water free of high mineral content nu nu mlI m a may be used Add water to each cell until the liquid level reaches the bottom of the vent well DO NOT sparslir Fmzing OVERFILLA IDTLL T Ln The engine should be opgated immediately after adding water particular in cold weather to I win hmm assure proper mixing of the water and acid bm 8 F The external condition of the battery and the cables Egg should be checked periodically The holddown should be kept tight enough to pre 32 2 vent the battery from shaking to prevent damage to 60339 the battery case It should not be tightened to the point where the battery case will be placed under a Discharge Cllomlcll Amlen severe Semin b It h Particular care should e taken to see t at t e A cell is discharged by completing an external cir top of the battery is free of acid film and dirt be cuit such as cranking a starter motor Sulfuric acid tween the battery terminals For best results when acting on both positive and negative plates forms a cleaning the battery wash with a diluted ammonia or new chemical compound called lead sulfate The sul soda solution to neutralize any acid present and then fate is supplied by the acid solution electrolyte The flush with clean water Care must be taken to keep acid becomes weaker in concentration as the discharge vent plugs tight so that the neutralizing solution does continues The amount of acid consumed is in direct not enter the cells proportion to the amount of electricity removed from To ensure good contact the battery cables should be the battery When the acid in the electrolyte is tight on the battery posts Check to be sure the ter partially used up by combining with the plates and minal clamp has not stretched This could cause the can no longer deliver electricity at a useful voltage clamp ends to become butted together without the battery is said to be discharged actually being tight on the post If the battery posts or The gradual weakening of the electrolyte in propor cable terminals are corroded the cables should be dis tion to the electricity delivered is a helpful action in connected by loosening the terminal clamp bolt and that it allows the use of a hydrometer to measure how removing the clamp with the aid of a puller Do not much unused acid remains with the water in the elec twist or pry on the cable to free it from the battery trolyte This information then can be used to deter post Clean the terminals and clamps with a soda mine approximately how much electrical energy is left solution and a wire brush After the cables are connec in each cell ted to the battery posts a thin coat of grease should be a lied The battery ground cable and engine to cham chlmluucuun crossliiiember ground strap also should be inspected The lead sulfate in the battery is decomposed by f rag d m and ndm n passing a current through the battery is a direction opposite to that of the discharge The sulfate is expel WARNING Explosive 0 are present mmm the led from the plates and returns to the electrolyte battery M all hmgS AUmd Openfhmes and SP ks thereby gradually restoring it to its original strength Hydrogen and oxygen gasses are given off at the ne8 BATTERY TESTING tive and positive plates as the plates approach the fully charged condition This is caused by an excess of When testing a battery perform the steps in the se charging current not totally accepted by the plates quence listed in the Battery Test Procedures Chart

 Next
Next